Europe style for Postpartum Glucose Test - UBREATH ® Spirometer System (PF280) – e-Linkcare
Europe style for Postpartum Glucose Test - UBREATH ® Spirometer System (PF280) – e-Linkcare Detail:
Reliable Result
Provides 6 parameters: PEF, FVC, FEV1, FEV1/FVC, EFE50, FEF75.
Accuracy and repeatability comply with ATS/ERS task force standardization (ISO26782:2009)
Complies with the ATS/ERS requirement for flow sensitivity down to 0.025L/s which is a vital characteristic for the diagnosis and monitoring of COPD patients.Portable Design
Hand-held device and easy to operation.
Automated BTPS calibration and free of environmental conditions influence.
Lightweight combines the benefits of portability.
Maintain easily and free daily Calibration.
Zero Cross-Contamination
Assured hygiene with disposable pneumotach gives NO authority to cross-contamination.
Patented design offers prevention.
Automated quality control and correction algorithm to minimize interference from operation.
User Friendly
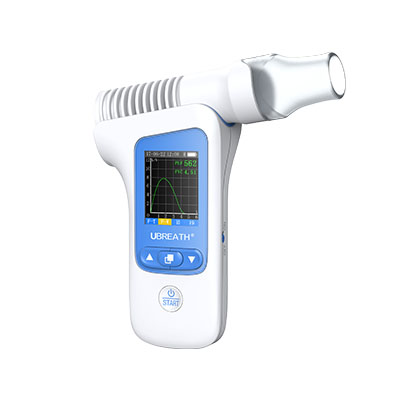
Incentive graph and digital indicators displayed supports doctors’ disease quick assessment.
Colorful range indicator allows quick assessment for better visual clarity.
Easily connect to PC for data exchange.
Data Transfer
Easily connect to PC via dedicated Bluetooth Communication Module for data exchange.
Access to UBREATH Software for more data analysis function.
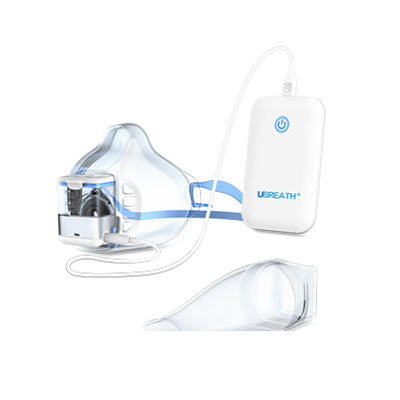
UBREATH Spirometer System(Model No. PF280)is a high-quality, easy to use, portable spirometer that delivers an ideal combination of portability, accuracy and safety. And it also helps doctor analyze the pulmonary data through V-T/F-V curve and digital indicator which is an ideal solution for the primary care, point of care, patients self-monitoring environment.
Technical Specifications
|
Feature |
Specification |
| Model | PF280 |
| Parameter | PEF, FVC, FEV1, FEV1/FVC, FEF50, FEF75 |
| Flow Detection Principle | Pneumotachograph |
| Volume Range | Volume: 0.5-8 L
Flow: 0-14 L/s |
| Performance Standard | ATS/ERS 2005 & ISO 26783:2009, ISO 23747:2015 |
| Volume Accuracy | ±3% or ±0.050L |
| Power Supply | 3.7 V lithium battery |
| Battery Life | Approximately 500 complete charge cycles |
| Printer | External Bluetooth printer |
| Memory | 495 records |
| Operating Temperature | 10℃ - 40℃ |
| Operating Relative Humidity | ≤ 80% |
| Size | Spirometer: 133x76x39 mm |
| Weight | 135g (including the Flow Transducer) |
Product detail pictures:
Related Product Guide:
Persisting in "High quality, Prompt Delivery, Aggressive Price", now we have established long-term cooperation with consumers from equally overseas and domestically and get new and old clients' large comments for Europe style for Postpartum Glucose Test - UBREATH ® Spirometer System (PF280) – e-Linkcare , The product will supply to all over the world, such as: Holland, belarus, Ottawa, As a way to make use of the resource on the expanding information and facts in international trade, we welcome prospects from everywhere on the web and offline. In spite in the top quality products we offer, effective and satisfying consultation service is supplied by our specialist after-sale service group. Solution lists and detailed parameters and any other info weil be sent to you timely for the inquiries. So please get in touch with us by sending us emails or contact us if you have any concerns about our firm. ou can also get our address info from our web site and come to our enterprise. or a field survey of our solutions. We're confident that we are going to share mutual results and build solid co-operation relations with our companions in this market. We're looking forward to your inquiries.
The company comply with the contract strict, a very reputable manufacturers, worthy a long-term cooperation.