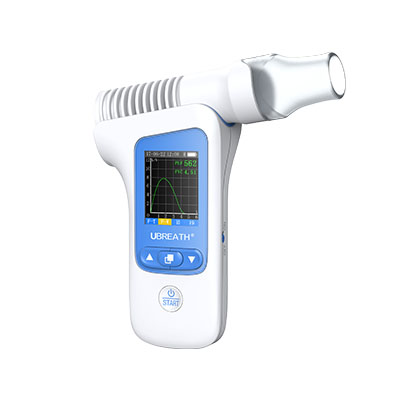
Factory best selling Fda Approved Cgm - UBREATH ® Exhalation analyzer (BA200) – e-Linkcare
Factory best selling Fda Approved Cgm - UBREATH ® Exhalation analyzer (BA200) – e-Linkcare Detail:
Features:
Chronic airway inflammation is a general feature of some types of asthma, cystic fibrosis (CF), bronchopulmonary dysplasia (BPD), and chronic obstructive pulmonary disease (COPD).
In today’s world, a noninvasive,simple, repeatable, quick, convenient, and relatively low cost test called Fractional exhaled nitric oxide (FeNO), often plays a role to help identify airway inflammation, and thereby support a diagnosis of asthma when there is diagnostic uncertainty.
Fractional concentration of carbon monoxide in exhaled breath (FeCO), similar to FeNO, has been evaluated as a candidate breath biomarker of pathophysiological states, including smoking status, and inflammatory diseases of the lung and other organs.
UBREATH Exhalation analyzer (BA810) is a medical device designed & manufactured by e-LinkCare Meditech to associate with both FeNO and FeCO testing to provide rapid, precise, quantitative measurement in order to assist with clinical diagnosis and management such as asthma and other chonic airway inflammations.
In today’s world, a noninvasive,simple, repeatable, quick, convenient, and relatively low cost test called Fractional exhaled nitric oxide (FeNO), often plays a role to help identify airway inflammation, and thereby support a diagnosis of asthma when there is diagnostic uncertainty.
| ITEM | Measurement | Reference |
| FeNO50 | Fixed Exhale flow level of 50ml/s | 5-15ppb |
| FeNO200 | Fixed Exhale flow level of 200ml/s | <10 ppb |
In the meantime, BA200 also provides data for the following parameters
| ITEM | Measurement | Reference |
| CaNO | Concentration of NO in the gas phase of the alveolar | <5 ppb |
| FnNO | Nasal nitric oxide | 250-500 ppb |
| FeCO | Fractional concentration of carbon monoxide in exhaled breath | 1-4ppm>6 ppm (if smoking) |
Product detail pictures:
Related Product Guide:
The company upholds the philosophy of "Be No.1 in quality, be rooted on credit and trustworthiness for growth", will continue to serve old and new customers from home and overseas whole-heatedly for Factory best selling Fda Approved Cgm - UBREATH ® Exhalation analyzer (BA200) – e-Linkcare , The product will supply to all over the world, such as: Afghanistan, Manila, Cancun, We set a strict quality control system. We have return and exchange policy, and you can exchange within 7 days after receive the wigs if it is in new station and we service repairing free for our products. Please feel free to contact us for further information and we will offer you competitive price list then.
Wide range, good quality, reasonable prices and good service, advanced equipment, excellent talents and continuously strengthened technology forces,a nice business partner.