Good News!IVDR CE Certification for ACCUGENCE® Products
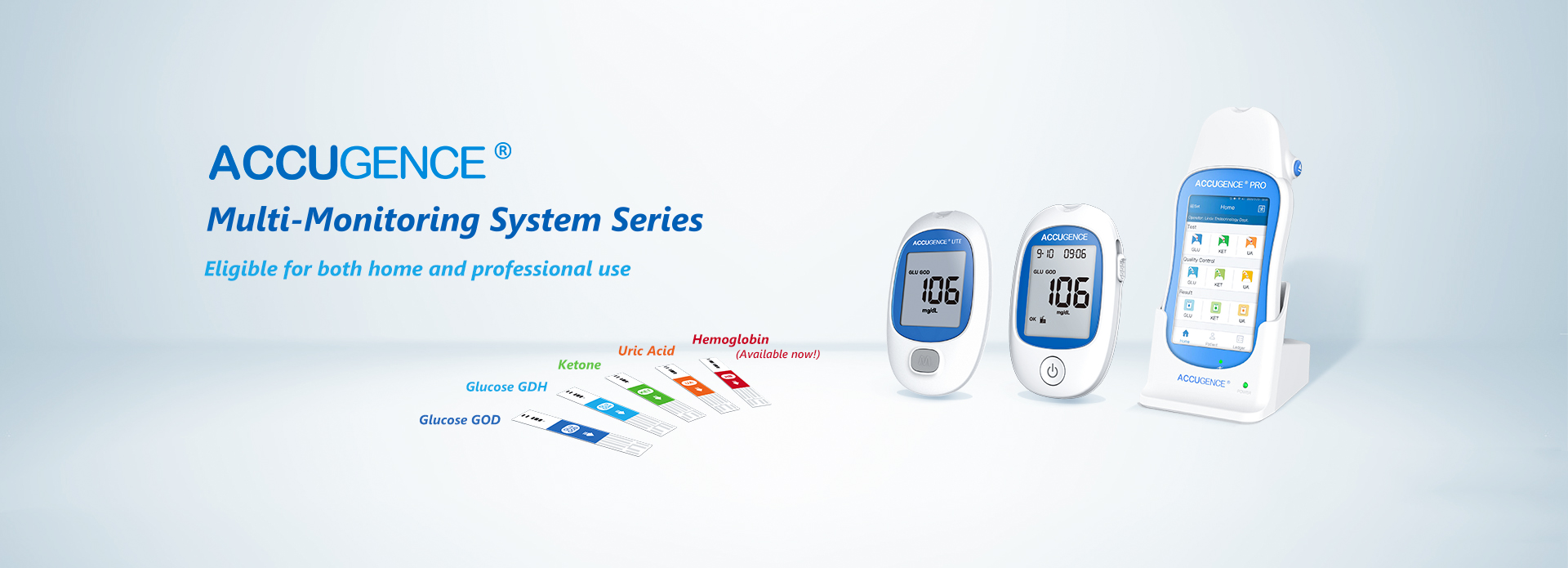
On 11th October, ACCUGENCE Multi-Monitoring System ACCUGENCE® Multi-Monitoring Meter (ACCUGENCE Blood Glucose, Ketone and Uric Acid Analysis System, including Meter PM900, Blood Glucose Strips SM211, Blood Ketone Strips SM311, Uric Acid Strips SM411, etc.) passed the Class C certification of IVDR.
By obtaining the IVDR CE certification issued by TÜV SÜD, the European Union’s notified body, which is an important and significant step in the progress of ACCUGENCE®, and marks a great breakthrough in the process of exploring the overseas market of e-LinkCare.
About IVDR
The EU In Vitro Diagnostic Medical Devices Regulation (IVDR), which came into effect on May 25, 2017 and implemented on May 26, 2022, has more comprehensive and stringent requirements for the technical review, clinical evaluation, and market supervision of in vitro diagnostic medical devices to ensure the safety, efficacy, and quality of the products.
According to the EU in vitro diagnostic medical device regulations, obtaining IVDR CE certification is a necessary condition for product access to the EU market, i.e., the product has obtained a “visa” to enter the European market.
The fact that our products can get the IVDR CE certification shows that our ACCUGENCE® Multi-Monitoring System has met the high standard requirements of the European Union market in terms of product quality, safety and effectiveness, as well as technical level, and also the quality control level has reached international standards.
Post time: Oct-25-2024